腫瘤免疫研究中可重復數(shù)據(jù)的“降本增效”方案
IMPROVING THE COsT-EFFICIENT GENERATION
OF REPRODUCIBLE DATA IN IMMUNO-ONCOLOGY sTUDIEs
Background
This case study compares PD-1, PD-L1 and CTLA4 antibodies from Leinco Technologies vs a competitor's products in a
series of in vivo mouse experiments. The objective of the study was to evaluate the in vivo efficacy of the various clones on tumor growth and tolerability.
The promise of immuno-oncology
The Nobel prize-winning research by independent groups headed by James P. Allison and Tasuku Honjo that led to the elucidation of cytotoxic T-lymphocyte antigen 4 (CTLA-4), and the programmed cell-death protein 1 (PD-1), respectively. This work has led to the development of a new class of anticancer agents called immune checkpoint inhibitors (ICIs).
The right tools for the job
About one third of the irreproducibility in preclinical research has been attributed to biological reagents and reference materials4. In the field of I-O, success depends on many factors, including access to informative animal models and high-quality reagents.Generating reproducible data in studies based on syngeneic mouse models ranging from days to several weeks depends on the availability of high-quality in vivo-grade antibodies. Low levels ofimpurities,demonstrated by extensive quality control (QC), minimize unwanted effects, especially in longer studies. Cost is also a factor since antibodies can contribute up to 10% of the budget, which means that securing a source of high- quality cost-effective antibodies can make quite a difference to the bottom line.
The Case Study below describes how one Contract Research Organization (CRO) investigated ways to carry out cost-effective reproducible science by securing an alternative source of in vivo-grade antibodies.
The Case Study
The business expansion of a syngeneic tumor model plat form at a CRO was threatened by data irreproducibility in in
vivo studies caused by lot-to-lot variation in murine monoclonalantibodies(mAbs) obtained from a supplier,Vendor A.They also faced significant increases in spending on antibodies and isotype controls. The need to improve
the cost-efficient generation of reproducible data led the research group at the CRO to evaluate LeincoTechnologies as apossible alternative supplier of antibodies with higher,more reproducible quality (see panel).The antibodies from LeincoTechnologies were also approximately half the price of those from Vendor A.
Evaluating the performance of antibodies
The researchers compared the in vivo efficacy and tolerability of clones of mAbs(anti-PD-1,anti-PD-L1,andanti-CTLA-4)and isotype controls from Vendor A with those from Leinco Technologies.To do this,they used the CT26 syngeneic
model,a murine colorectal carcinoma derived from BALB/c mice that is one of the most commonly used murine solid tumor models and is categorized as a highly immunogenic tumor model.
Table 2: Antibodies and isotypes tested
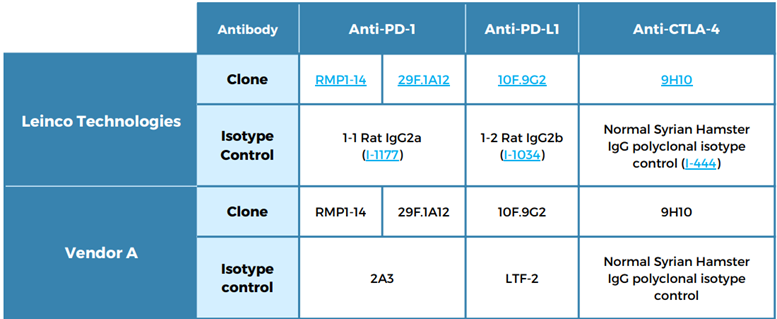
Testing involved groups of eight mice, each dosed at 10 mg/kg (0 for PBS control) per intraperitoneal administration, with twice-weekly (BIW) dosing for three weeks.
High quality functional-grade antibodies
Leinco Technologies has developed proprietary methods for manufacturing in vivo functional grade antibodies with the highest purity standards in the industry. In vivo ***TINUM? functional grade antibodies have the most stringent quality specifications for purity, endotoxins, aggregates, and leachable protein A (Table 1 and Figure 1). Each antibody is available in custom concentrations and package sizes. In vivo ***TINUM antibodies are also pathogen free and tested by the IDEXX Impact I PCR mouse pathogen profile.
Table 1. Qualitative ability of antibody pairs to detect Influenza A, H1N1, strain AWS33 by lateral flow test
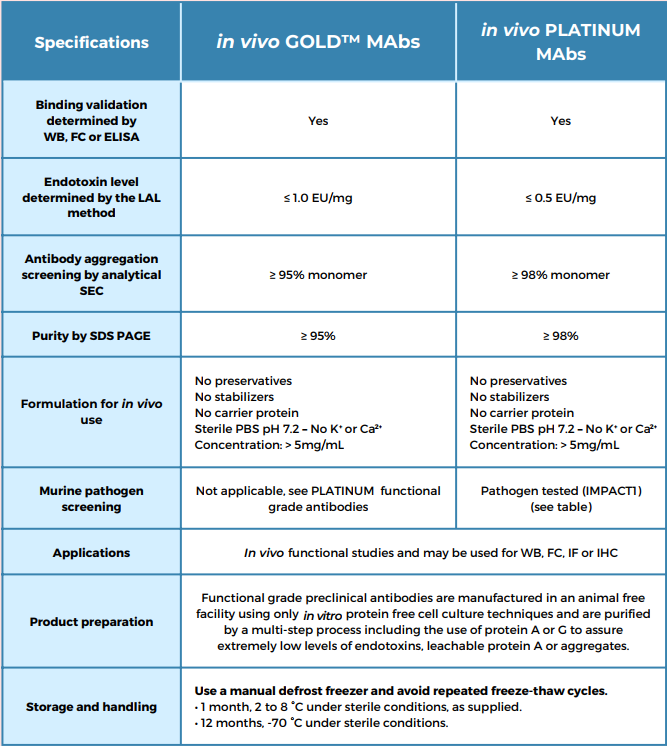

Figure 1. Quality control of in vivo antibodies: RMP1-14In vivo antibodies from Leinco Technologies are subjected to extensive QC to ensure high reproducibility between lots. The example in Figure 1 shows the results for different lots of RMP1-14 antibody, directed against PD-1. The lots have consistently high purity as indicated by non-reducing and reducing SDS-PAGE (Fig. 1A and 1B, resp.). Analysis by size exclusion chromatography gave superimposing chromatograms for all nine lots, with overlapping retention times and minimal baseline noise (Fig. 1C). Endotoxin data for eight lots indicated that all lots were below the specification of ≤ 0.5 EU/mg as determined by the limulus amebocyte lysate (LAL) method (Fig. 1D).
Results
Anti-PD-1Both anti-PD-1 antibody clones (RMP1-14 and 29F.1A12) from Leinco Technologies were more efficacious than Vendor A's products (Figure 2).
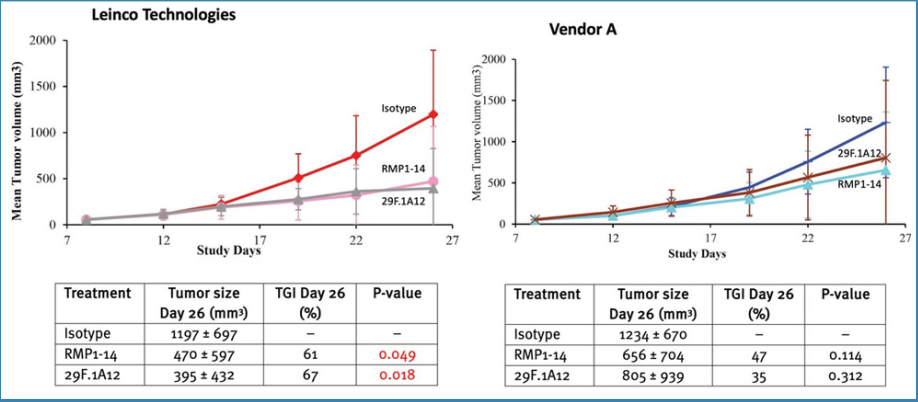
Figure 2. Efficacy of anti-PD-1 vs isotype
Anti-PD-L1In the case of anti-PD-L1, 10F.9G2 clones from Leinco Technologies and Vendor A showed similar antitumor activity, with tumor growth inhibition (TGI) of 47% and 59%, respectively (Figure 3). The efficacy of Vendor A anti-PD-L1 10F.9G2 was in line with historical data generated at Leinco Technologies.
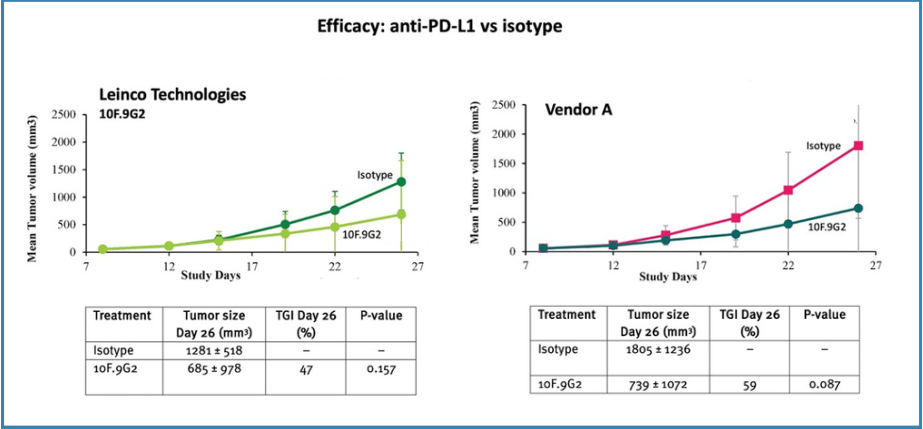
Figure 3. Efficacy of anti-PD-L1 vs Isotype
Anti-CTLA-4
The anti-CTLA-4 clone 9H10 from Leinco Technologies was significantly more efficacious than the same clones from Vendor A (Figure4).
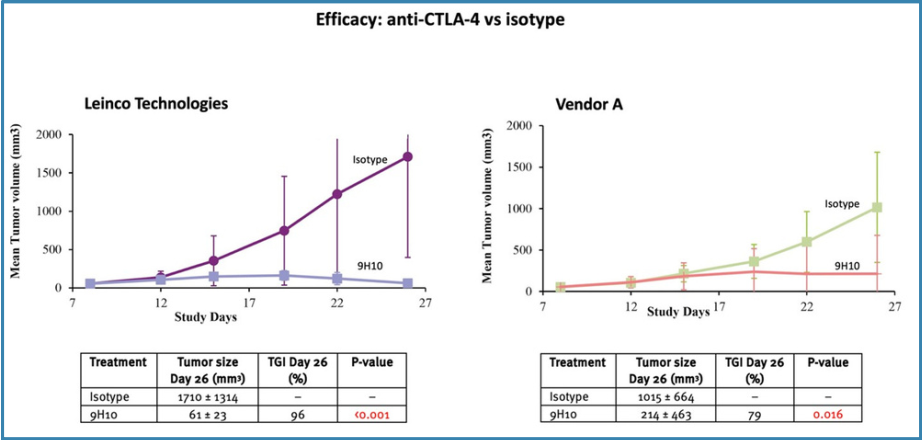
Figure 4. Efficacy of anti-CTLA-4 vs Isotype
Efficacious antibodies for cost-effective I-O studies
The Case Study confirmed that antibodies from Leinco Technologies were more efficacious inin vivo studies than those from Vendor A:Anti-PD-1: Clones of anti-PD-1 antibodies (RMP1-14 and 29F.1A12) from Leinco Technologies were moreefficacious than Vendor A's products (x1.3 and x1.9 higher TGI, respectively).Anti-PD-L1: 10F.9G2 clones from Leinco Technologies were slightly more efficacious than Vendor A's, with TGI 47% and TGI 59%, respectively.Anti-CTLA-4: Clone 9H10 from Leinco Technologies was more efficacious than Vendor A's products (x1.2 higher TGI).
This performance, backed by extensive QC, combined with the possibility of reducing R&D costs were powerful incentives to change supplier.
There is, however, always room for improvement. The reproducibility and accuracy of preclinical modeling is also affected by the species used to prepare antibodies. Investigations have revealed that using non-murinized (e.g., rat- based) antibodies can result in xenogeneic responses, which do not occur clinically, and may also fail to recapitulate the clinical efficacy and toxicity of single agent checkpoint inhibition . This is an issue that Leinco Technologies is currently addressing in our efforts to provide best-in-class in vivo-grade antibodies for I-O studies.SummaryGenerating reproducible data from immune-oncology in vivo studies depends on many factors, including the availability of high-quality antibodies.Antibodies from Leinco Technologies for in vivo use are supported by extensive QC data.An independent study comparing the in vivo efficacy on tumor growth and tolerability of various clones showed that antibodies from Leinco Technologies had superior performance compared to those from another supplier.Antibodies from Leinco Technologies support cost-efficient generation of reproducible data from animal models in the study of immuno-oncology.